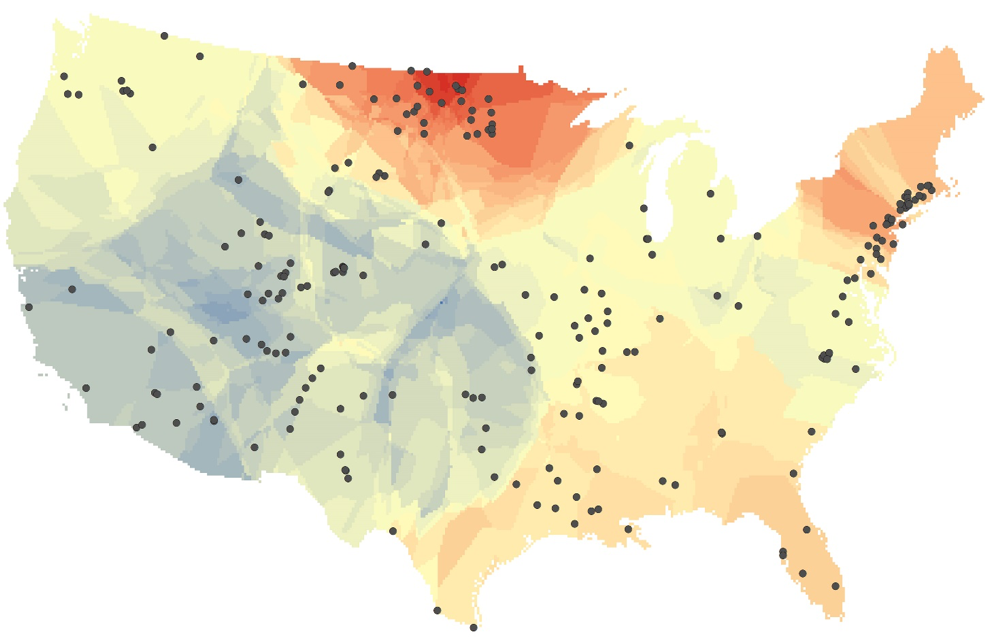
Legend for map: Changes in salinity of U.S. inland waters where warmer colors indicate increasing salinity and cooler colors decreasing salinity. Dots are USGS sampling sites (Map by Ryan Utz, Chatham University).
I remember when my parents bought a water softener after a family move. We had never had one before and I wondered why we needed a large contraption to treat the water coming into the house. I learned water softeners removed salts from “hard water” – think rich in dissolved minerals. The softening enables a better “froth” when using soaps. Water softeners also help reduce excessive scale build-up in pipes, preserving plumbing.
Natural freshwaters are characterized as being “hard” and “soft”. Extremely soft water is almost like distilled water with low concentrations of dissolved chemicals. You might find such water in a high mountain lake or stream. Extremely hard water – typical in arid regions – is full of minerals due to high evaporation rates that concentrate chemicals in the water.
Hardness and softness, however, are not fixed in time or space. For example, hardness can vary among wells in a local area. Hardness can also change in surface waters over time as we learned in a study recently published in Proceedings of the National Academy of Sciences[1]. We analyzed data from 232 rivers and streams regularly monitored by the United States Geological Survey. We asked if there are long-term trends in dissolved salts as well as the alkalinity of rivers and streams. Alkalinity is the opposite of acidity and reflects low concentrations of acids relative to bases.
A word on alkalinity, which is not a water issue we often think about, but one important to our daily lives. Many cleaners are alkaline and this gives them “oxidizing” ability to dissolve dirt, grease, grim, and stains. Highly alkaline waters are problematic because they are caustic and because some chemicals like ammonia become much more toxic. Most aquatic organisms cannot take highly alkaline conditions.
Surprisingly, our study found both salinity and alkalinity are increasing in flowing waters of most of the eastern and central U.S. Chemically, this means the concentrations of dissolved salts such as calcium carbonate and sodium chloride are going up. We estimated that about 40% of the surface of the contiguous U.S. has increasing concentrations (see map). We dubbed this joint increase in salinity and alkalinity as – the freshwater salinization syndrome.
Why are many U.S. waters getting salty and more alkaline? There are several reasons. De-icing road salts spread on highways dissolve and run off. Fertilizers including lime, often applied to reduce soil acidity, are used widely and likely contribute to increased inputs. Rivers are replete with structures like concrete bridges and docks that slowly erode and dissolve, providing another source of inputs. Geological weathering of natural landscapes is accelerated by air pollution – meaning rocks are “melting” (dissolving) faster. Finally, land use change including urbanization leads to greater direct run-off into surface waters. Urban runoff is enriched with common salts. In aggregate as humans have transformed landscapes by developing cities, suburbs, and farms, we have caused greater inputs of salts and more alkaline conditions in our freshwaters. An exception to these trends is that in the western U.S., salt and alkalinity tend to be declining (see map). This may reflect the positive effects of water management now common in arid western basins.
Salty, alkaline freshwater can cause at least three problems. First, the quality of drinking water is reduced. For those with dietary restrictions (like low sodium diets), use of tap water might even be restricted. Second, water chemicals affect pipe corrosion and may lead to undesirable changes that require treatment, or in the extreme, restrict water use because of increased concentrations of lead, copper, or other toxic metals. Third, salty water is bad for aquatic life adapted to soft water. Species sensitive to saltiness may be lost and only salt-tolerant organisms survive.
This problem has remedies. Road salt can be reduced without compromising safety. Lower applications, alternative chemicals, and pre-storm road treatments are viable strategies for reducing road salt. Fertilizers can be often be reduced as well and buffered against running off into streams and rivers. There are many so-called “best management practices” designed to keep the substances in fertilizers out of waterways. Urban areas and roadways can be managed better with green infrastructure (plantings), greater infiltration, and improved drainage to reduce run-off laden with salts.
There is no water softener for natural waterways. We need to reform land use and to redesign developed areas to reduce inputs. These changes can bend the upward trends of chemicals entering our waters and restore fresh, pure water upon which much of life depends. There are many good reasons to make these changes. Salty, alkaline water is one more.
[1] Kaushal, S.S., G.E. Likens, M.L. Pace, R.M. Utz, S. Haq, J. Gorman, and M. Grese. Freshwater salinization syndrome on a continental scale. Proceedings of the National Academy of Sciences, U.S.A., Early Edition: 1-10.